
Laboratory Data
Management
System
LDMS is a comprehensive laboratory information management system for managing collections of biological specimens. It is used by clinics and laboratories around the world to manage specimen tracking, inventory storage, specimen shipment, barcode labeling and much more.
LDMS was developed and is maintained by Frontier Science, a not-for-profit organization that has over 40 years of data management experience.
What We Offer
LDMS is a comprehensive LIMS solution for laboratories, repositories, and clinical trial networks to manage biological research specimens. This cloud-based software requires zero installation and is accessible anywhere you have a stable internet connection. Clients know that their biological specimen data is secure; LDMS is developed, maintained, and supported by Frontier Science Foundation, an expert in data management and regulatory compliance.
LDMS clients also receive concierge-level service with continuous technical support and robust, study-specific end-user training, not just support at setup.
For additional specialization, collaborating with Frontier Science Foundation as your Data Management Center allows for LDMS customization, tailored project solutions, and study-specific data management.
Specimen Inventory
Efficiently log and track your specimen inventory using our intuitive user interface. Define custom specimen collection templates to standardize your data entry and minimize manual input. Quickly locate records in your database to review work or apply updates using a comprehensive set of record filters or 1D/2D barcode scanning capabilities.
Storage
Powerful and intuitive storage management features keep your specimens organized. Easily build and customize your unique, virtual storage environment aligned to your physical freezers. Assign specimens to individualized storage positions and locate specimens on-demand using a comprehensive set of record filters and robust reporting capabilities.
Shipping
Efficiently generate manifest reports and data- transfer files to move inventory between labs. Build shipments of specimens by using our comprehensive filters, bulk uploading lists of specimens, or leveraging your LDMS storage environment to quickly assign stored specimens for shipping.
Barcodes and Labels
Customizable features allow you to define and standardize unique specimen labels. Critical specimen information, including unique identifiers and 1D/2D barcodes, is displayed on your labels for each project. Label generating capabilities are integrated throughout the application, giving flexibility to your workflow and allowing you to decide when to print.
Data Exchange
Easily extract your data using LDMS reporting capabilities and our integrated API support. Utilize dozens of predefined and formatted reports across a variety of administrative and data categories or use our Custom Report Builder to define reports that meet your specific criteria. Go beyond basic reporting and use our integrated API to stream your LDMS data into larger and more complex data workflows.
No Installation Needed
LDMS is a cloud-based application that does not require software installation or a dedicated server; simply log-in to your user account at webldms.org through your supported browser of choice. Once your LDMS license is live, our User Support team will set up your LDMS Laboratory Database and activate your users within 72 hours.

Technical Support
Our User Support team is committed to providing fast and effective solution-oriented assistance. User Support is available by email or phone to answer your questions about using LDMS, diagnosing and troubleshooting problems, user account access management, and helping your laboratory get the most out of your LDMS database.
Training Resources
The LDMS Training Team and Frontier Science offer our end-user several different styles of educational training opportunities. Your team can choose from General and Project-specific webinars, On-Site training at your laboratory location, or independent study / self-training through our curated recorded webinars and content examinations with certificate generation.

Validation Resources
The software validation process confirms that the software product functions as intended. LDMS is fully validated under FDA 21 CFR Part 11 and is hosted in a FISMA moderate, SOC-2 certified environment. This ensures accuracy, reliability, and consistent performance. End-users can request Frontier Science developer validation documentation and end-user validation templates.
LDMS is Worldwide
LDMS is used across the globe to help manage
specimen inventory for a range of laboratories.
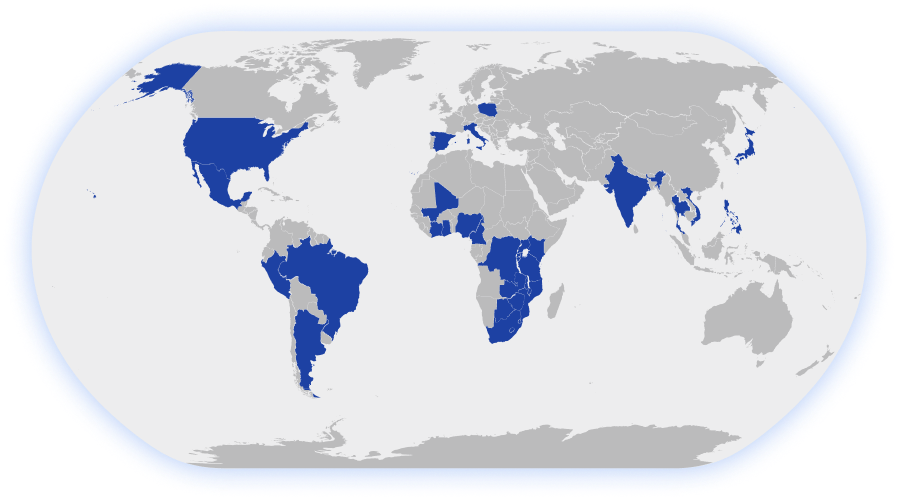
30+
Supported Countries
300+
Laboratories use LDMS
1000+
Active LDMS Users
Projects
With our unique expertise, affordability, and proven data management software, LDMS is the trusted choice for simplifying workflows and standardizing data collection to put your research on the path to success.
Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections
Cancer Prevention Clinical Trials Network

Clinical Pharmacology Quality Assurance

HIV Prevention Trial Network

HIV Vaccine Trials Network

International Maternal Pediatric Adolescent AIDS Clinical Trials Network

MACS/WIHS Combined Cohort Study

Pediatric HIV/AIDS Cohort Study

Population-based HIV Impact Assessment
Announcements/Release Notes

Have More Questions?
We’re here to help and answer any questions you might have. Please contact us and a member of our User Support team will be happy to assist you.
