With LDMS 11.1 many changes were made to the pharmacology (PK) assay feature in LDMS.
Some of the updates will not be made available until CPQA and associated Network Leadership has determined the most appropriate way to make use of the specific features. These restricted features include the ability to log and track Calibration Standards and QC Lot Controls and the ability to capture results for run types such as calibration, stability, matrix, validation, and proficiency.
Other features will be available immediately after you upgrade to LDMS 11.1. Some of these changes are as follows:
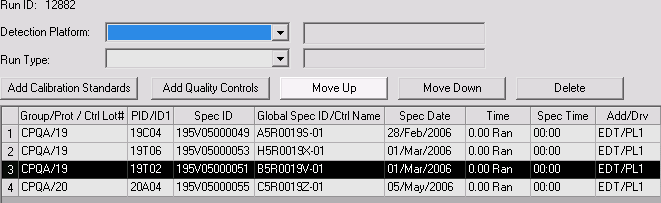
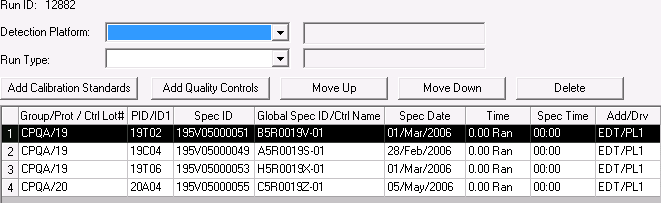
The ability to rearrange items added to a PK run. The Move Up and Move Down buttons on the preview tab will allow the user to rearrange items in the grid by moving them up or down one position at a time.
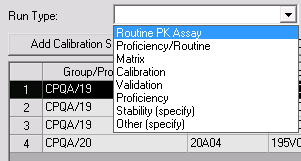
The user must select a run type before completing a run. This new selection contains run types such as Routine, Proficiency, Validation, Stability, etc.
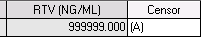
The A censor will now be automatically calculated by LDMS.
- LDMS will compare the result entered to the design upper limit for this run. If the result entered is above the upper limit for the assay LDMS will automatically assign the (A) censor.
- The user will no longer be able to manually assign the (A) censor
- It is expected that (A) censored results will be diluted and run again
- To avoid getting an (A) censor on the diluted result, it is expected the user will enter the new diluted result, assign the (U) censor, and specify the dilution factor.
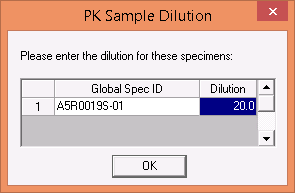
The ability to assign a dilution factor to diluted specimens. When applying the U censor to a diluted sample LDMS will now prompt the user to specify the dilution factor as 1x #.
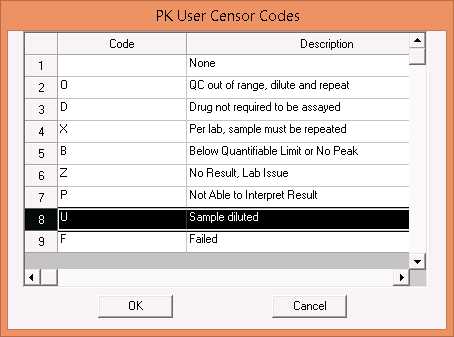
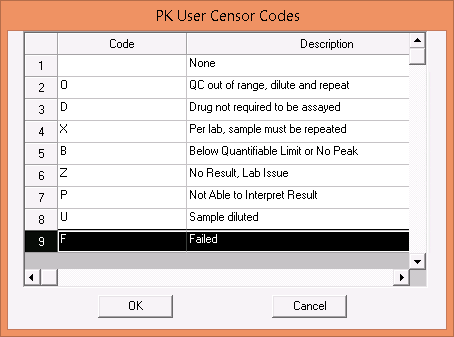
The user can now manually flag a specimen as failed with the F user censor.
The user no longer needs to specify the Run Upper and Lower Limits.
- The Run Upper and Lower Limits will always be assumed to be the design templates lower and upper limits.
- The user will not need to specify individual upper and lower limits every time a PK assay is completed. This will effectively make the (L) censor code obsolete for new runs.
