Frontier Science is pleased to announce that many new enhancements have been made to LDMS for the web (version 5.0).
Here are are a few highlights from these changes, as well as the full release notes.
Highlights
Improved filtering
Filtering is available in several places throughout LDMS and is a handy way to quickly find a specimen.
As new filters have been added, the list has gotten rather long. To save space, filters can now be collapsed.
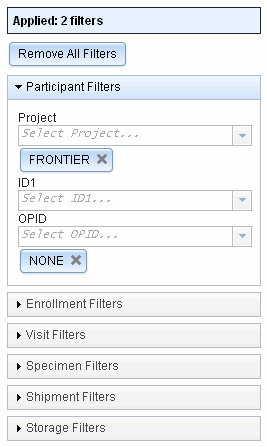
We’ve added many new filters, such as import date. You can also now use filters on the Shipment History page.
We’ve made a number of changes to how filters work on a technical level too. Applying and removing filters should now be significantly faster.
Improvements to Editing Multiple Aliquots
Editing multiple aliquot specimens at once has now been made more intuitive.
Sometimes you may want to change several aliquot specimens at once. On the Specimen Management page, you can now do this by pressing and holding Ctrl or Shift while selecting aliquots. Now when you click Edit, you’ll be modifying all of the selected specimens.
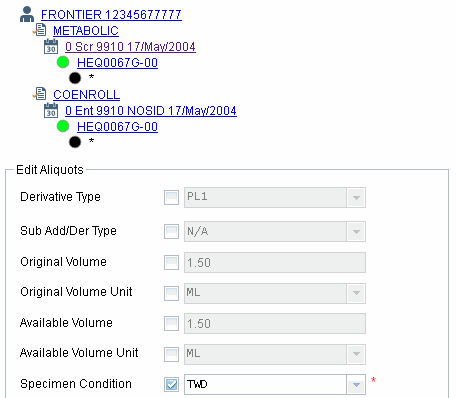
Selecting the check box means that you are going to change that item for all of the specimens that were selected. This is handy when you need to assign the same condition or comment to multiple specimens.
Assigning Tests to Specimens
We are still working on adding assay support to the web version of LDMS. In the mean time, you can now view test assignments for specimens, assign additional tests, and flag tests that are not expected to be completed.
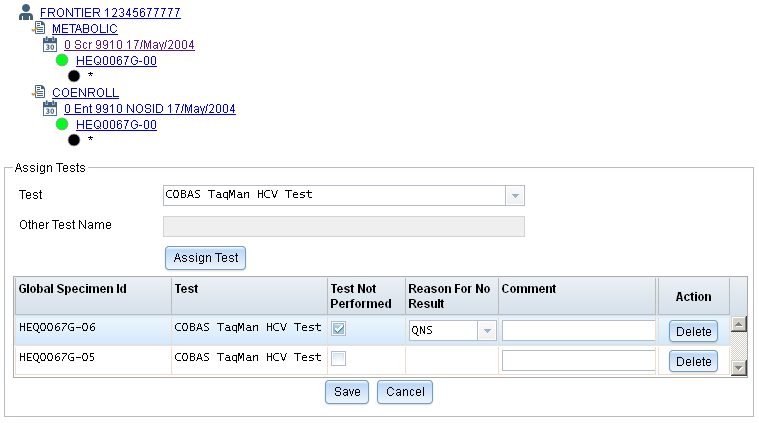
While you cannot add assay results in LDMS for the web yet like you can in LDMS for Windows, you can indicate a Reason for No Result if you already know that the test will not be run. Tests can be assigned to multiple specimens at once by pressing and holding the Ctrl or Shift keys.
Test assignments are included when you ship specimens between LDMS laboratories.
Label Printing
We’ve made many improves to the way label generation works. The page for generating labels across participants and specimens has been completely redesigned.
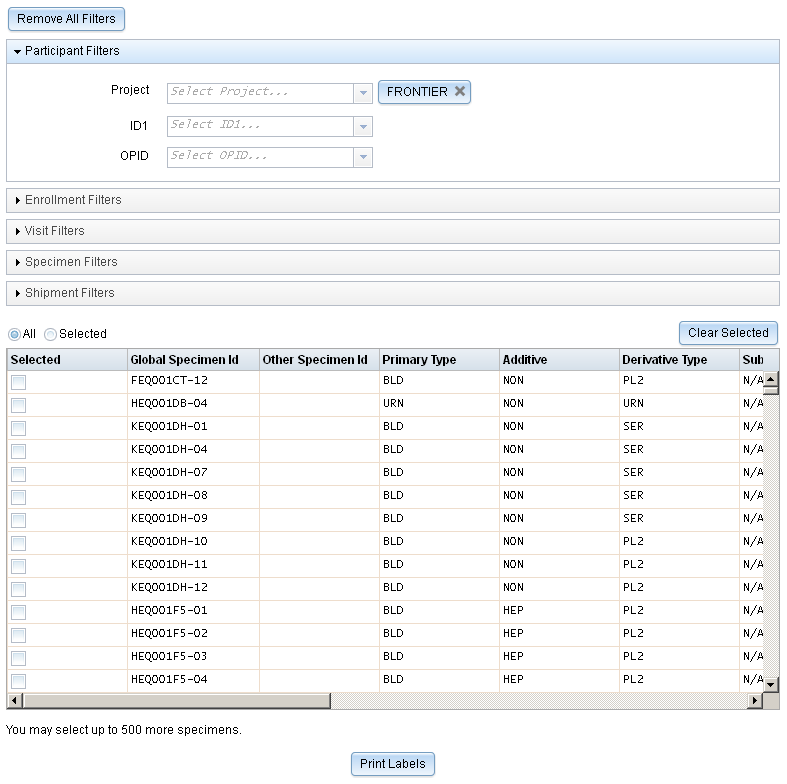
You can now use filters to narrow down a list of specimens, and then select the specimens for which you want labels. This allows much finer control over the labels that will be generated compared to the previous process, where you needed to manually add specimen criteria and any specimens that met that criteria were included.
Creating Storage Templates
When creating new storage items, you can now choose to save the item that you are creating as a new template. This will allow you to quickly re-use the same storage item again.
Full Release Notes
There have been over 100 improvements and fixes to this version of LDMS. The full list of changes in this release is below.
- Added ability to remove specimens from storage on the Specimen Management page. (R-07047)
- Added specimen and storage comments as available fields to display on reports in the Custom Report Builder. (R-15509)
- Fixed rounding issue with certain controls. (R-15726)
- Added position to confirmation messages for deleting storage items. (R-16371)
- When creating storage items, added ability to save the confirmation as a new storage item template. (R-16545)
- Fixed issue where adding/removing rows on forms caused validation errors. (R-16953)
- Import date is now displayed when viewing specimens on the Specimen Management page. (R-17835)
- Users are now asked to confirm shipment temperature when receiving a shipping file, and will be warned if that temperature differs from the temperature indicated by the sending laboratory. (R-17863)
- Fixed issue where it was possible to add a specimen to a shipment that is already in a shipping container. (R-18578)
- Fixed issue that could cause performance problems. (R-19504)
- Shipping files will now be structurally validated when they are created. (R-20867)
- When attempting to add items to a storage unit template, you will automatically be prevented from inadvertently adding more items than the unit’s capacity. (R-21048)
- When receiving specimens in a shipment, if any aliquot specimens have identical specimen information, they will now be grouped under the same parent primary specimen. (R-21116)
- It is now possible to select a condition when adding specimens using the Quick Add feature. (R-21436)
- When receiving a cross LIMS shipping file, the type of file will now be correctly shown as “Cross-LIMS.” (R-21482)
- The grid used on QA/QC screens is now consistent with grids used on other pages. (R-21778)
- In the filter pane, each filter category can now be collapsed to save space. (R-22101)
- Barcode scanner input is now correctly handled if a user has a text box selected. (R-22165)
- When selecting storage items to ship, users can now select multiple storage items at the same time. (R-22298)
- When receiving a shipment, specimens will now be flagged as unavailable if the specimen has one of several conditions that automatically make a specimen unavailable. (R-22300)
- Users can now consolidate storage containers to eliminate empty spaces. (R-22362)
- Users can now use import date as a standard filter on several pages. (R-22988)
- Users can now use filters on the Shipment History page. (R-22995)
- When a specimen is part of a pending shipment, a link to that shipment is now displayed when viewing details for that specimen. (R-23219)
- When assigning a storage location to specimens, more information about available specimens will be displayed. (R-23225)
- The interface for pages used for printing labels and defining labels formats have been improved. (R-23226)
- Project-specific labels for participant (ID1), protocol (ID2), and additional (ID3) identifiers are now more consistently used. (R-23293)
- Fixed issue where the incorrect container location was displayed when previewing an incoming shipment. (R-23366)
- Participant identifier, visit value, and cell volume now appear correctly on shipping manifests. (R-23745)
- If users attempt to add a participant that already exists, they will now be given the option to view that participant. (R-23805)
- The TIES preset project is now available. (R-23932)
- Fixed issue where column headers appears on the graph page of the Cell Yield QA/QC Summary, Time to Freeze QA/QC Summary, and Time to Process QA/QC reports. (R-23988)
- Fixed issue with session time-outs and dialog windows. (R-24026)
- Fixed erroneous use of the “box” instead of “container.” (R-24030)
- Fixed issue where white space in certain controlled input boxes was not compressed correctly. (R-24038)
- Fixed issue where collection date was not validated correctly in the Manage Enrollments window. (R-24048)
- Fixed issue where long comments that could not be saved on the Quick Add page would be cleared before allowing the user a chance to shorten the comment. (R-24051)
- Fixed issue where attempting to perform QA/QC on a shipment without specimens did not fail appropriately. (R-24058)
- Fixed issue where trying to generate a report with a criteria sentence containing angle bracket pairs failed. (R-24068)
- Changing filters will now no longer cause a full page refresh, improving page performance. (R-24104)
- Reports will now show specimen and storage information at the time a specimen was sent or received, rather than its current state. (R-24146)
- Additional specimen, shipment, and storage filters have been added. (R-24149)
- Added ability to assign tests to specimens. (R-24178)
- To avoid confusion, setup and ship dates are no longer displayed to the user for shipments that have been received. (R-24215)
- Fixed issue where date filters were incorrectly treated as required fields. (R-24237)
- Fixed issue where first and last participant navigation buttons caused the page to refresh, even if the user was already viewing the first or last participant. (R-24239)
- If a user attempts to access the Select Laboratory page when not signed in, they will be appropriate re-directed once they do sign in. (R-24261)
- Clarified error message shown when a user attempts to sign in using an expired temporary password. (R-24273)
- Added support for containers larger than 26 × 26. (R-24294)
- It is now possible to manually set the initials of the person that performed shipment QA/QC. (R-24300)
- Fixed issue where it was not possible to enter IPK information using the Quick Add feature. (R-24360)
- It is now possible to receive shipments that contain IPK data. (R-24361)
- Improved the usability of editing multiple specimens at the same time. (R-24362)
- Fixed issue where storage items were not appropriately removed if a shipment was un-imported. (R-24372)
- Improved control used to select specimens for shipping and storage. (R-24452)
- Corrected AMP label format. (R-24530)
- Fixed issue where shipped primaries could erroneously be flagged as available even if aliquots were created. (R-24604)
- Test assignments can now be shipped in CSV format shipping files. (R-24763)
- Fixed issue where global specimen IDs were cut off on the Specimen Processing Report. (R-24950)
- Corrected label for additional time on the QA/QC page. (R-25073)
- Test assignments can now be shipped and converted from Oracle LDMS databases. (R-25082)
- Improved speed of importing shipments that contain a large number of specimens (over 5,000). (R-25281)
- Participant identifiers for HPTN, MTN, and VTN are now checked to ensure they are 9 or 10 characters. (R-25310)
- Increased allowable time for requests to reduce time-out errors on processes that can take a long time, such as importing a shipment. (R-25329)
- When a time-out error occurs, the user will now be given the option to clear active filters and try to load the page again. (R-25330)
- Improved the speed of saving pending shipments. (R-25391)
- Improved the speed of saving specimens using Quick Add. (R-25392)
- Improved the speed of shipping storage items. (R-25393)
- Fixed issue where some specimens could be removed from a shipment if the shipment was un-shipped. (R-25436)
- Fixed submission errors that occasionally occurred when attempting to clear all active filters. (R-25449)
- Fixed issue where multiple SHIPPING IMPORT FREEZER storage units could be created. (R-25465)
- Fixed issue with filtering specimens by specimen ID. (R-25521)
