Frontier Science is pleased to announce that many new enhancements have been made to LDMS for the web (version 6.1).
Here are a few highlights from these changes, as well as the full release notes.
Highlights
New Reagent Logging Module
The reagent logging module can be used to manage reagent lots for the laboratory at an
administrative level.
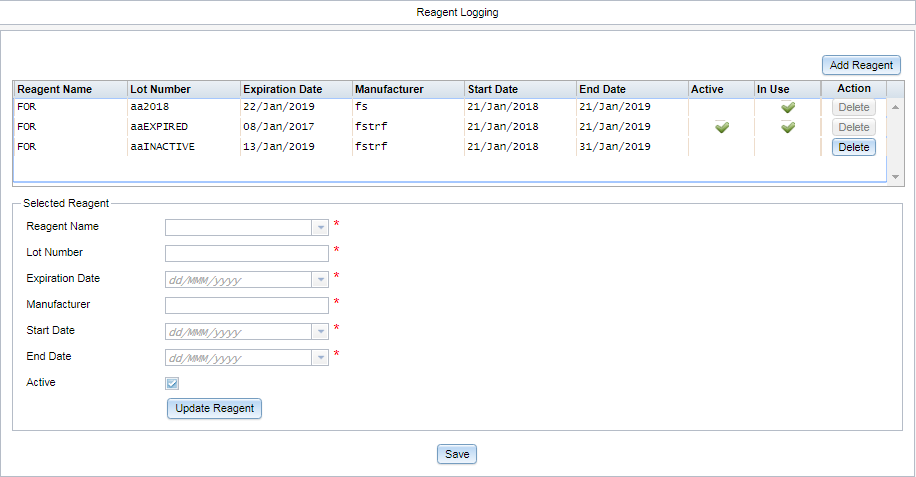
Users can assign reagent lots to primary and aliquot samples in Specimen Management or via QuickAdd. Options are limited
to the lots that have been defined in the reagent logging module.
Workflow Improvements
Users are now able to upload a list of global specimen IDs defined in a text file for various features. These include:
- The Labels page: uploading the file on the Labels page will set a specimen criteria to locate any sample with a global ID appearing in the list.
- The Storage page: The settings filter to the storage tree now has an option to upload the list which will set a specimen criteria to locate any sample with a global ID appearing in the list.
- The Shipping page: When adding loose samples to a shipping container, the filters have an option to upload the list of global specimen IDs. Doing so there will also set a specimen criteria to locate any sample with a global ID appearing in the list.
New HPTN PTID Correction Procedure
A check has been added to specimen management that prevents PTID changes for existing (saved) HPTN records. PTID changes
in the LDMS require approval from the HPTN network. HPTN PTID changes can no longer be made without a challenge code
response.
Improvements to Storage
Additional checks have been implemented to prevent the accidental deletion of storage containers or templates and samples.
The Web LDMS move, bulk add, and delete storage reports are now available in a single UI where the user can select
which action type(s) they would like to include on the report. The user has the option to include actions from this
particular session or from a specified date range.
Additionally,the following features have been added to the application:
- Added ability to set frozen date and time when storing samples
- Added optional second condition code
- Added flag for SCHARP projects to indicate samples not collected per protocol
- It is now possible to perform qa/qc on “large” shipments (1000 samples) without receiving an error
Full Release Notes
There have been over 60 improvements and fixes to this version of LDMS. The full list of changes in this release is below:
- Deleting freezer templates now prompts user for confirmation (R-17574)
- Shipment format defaults to LDMS for new shipments (R-17886)
- Users now able to upload a list of global specimen IDs in text file for various features (R-19515)
- Shipment history level detail window’s contents now prevents editing (R-20885)
- Specimens Not in Storage report status field and filter criteria removed (R-21624)
- Fixed a bug where QuickAdd updates Processing Date when Draw Date changes (R-21760)
- Users can now delete primary and its associated specimens. A message will prompt the user to confirm the deletion (R-21999)
- URL queries using ID1/ID2/ID3 now encode the query parameters (R-22084)
- The Web LDMS move, bulk add, and delete storage reports have been made available in a single UI where the user can select which action type(s) they would like to include on the report. The user has the option to include actions from this particular session or from a specified date range (R-22271)
- Views are now compiled during a nightly build (R-22341)
- Fixed unsafe Regex usage (R-22351)
- Added ability to set frozen date and time when storing samples (R-22550)
- Fixed error when cascading Collection Date to Received Date (R-22855)
- Added option to combine patients and enrollments to Web LDMS (R-22991)
- Fixed inconsistent naming of Shipment Date (R-23612)
- Patches can mark themselves as never needing to run from the pre-patch check (R-24694)
- Imported unsupported tests from Oracle (R-25086)
- Shipping history now stores Global Spec ID assigned during import (R-25316)
- Fixed Patient Editor showing incorrect ID1 label after failed edit (R-25766)
- Fixed CSV import failing when trying to parse an invalid Global Spec ID (R-25824)
- Generation of labels for shipped, unavailable samples now allowed (R-26227)
- Dimpy lists all constraint violations after failed import (R-26351)
- Improved automated reporting for login information when requesting support from FSTRF (R-26689)
- Error message text made clearer (R-27122)
- Fixed error trying to delete a freezer/level/box with set conditions for specimens that has actually already been deleted (R-27242)
- New specimen management check prevents deletion of a sample that is on a non-deleted Test Result Run and provides error text explaining why (R-27378)
- QuickAdd ignores backspace unless a text input is selected (R-27504)
- Added optional second condition code (R-27549)
- Added second condition code option to Custom Report Builder (R-27550)
- Added reagent logging module (R-28200)
- Added flag for SCHARP projects to indicate samples not collected per protocol (R-28212)
- Fixed Batch Storage Report erroneous error message about report not being available after shipping. Also fixed report so it doesn’t show primary samples that weren’t actually shipped (R-28415)
- It is now possible to perform qa/qc on “large” shipments (1000 samples) without receiving an error (R-28421)
- Added message when a connection error occurs suggesting that the user rescan the databases on that server (R-28441)
- Added ability to edit and save invalid frozen date in QuickAdd (R-28511)
- Barcode scanning in specimen management will now properly report that the “specimen with Global Spec ID $X is outside of the current filter criteria” when it is filtered out by primary or aliquot filters. (R-28515)
- Web LDMS upgraded to Visual Studio 2015 (R-28525)
- Added ability to set frozen date and time and Processing Tech Initials for an entire storage (R-28549)
- Created new maintenance web page to display during Web LDMS patching process (R-28564)
- When a primary is co-enrolled, it’s superior enrollment can now be correctly changed between the co-enrollments (R-28634)
- Added check to specimen management that prevents PTID changes for existing (saved) HPTN records (R-28658)
- Web LDMS will now prevent aliquots from being added to an unaliquoted primary that was sent on an outgoing shipment (R-28660)
- Keyboard navigation on storage tree now works correctly (R-28693)
- Fixed specimen ID field on shipping manifest getting cut off by global ID field (R-28695)
- Fixed user permission summary in account manager to accurately reflect assigned permissions (R-28809)
- Specimen log report now counts the number of aliquots correctly (R-28891)
- Web LDMS WIHS processing log report updated to account for new Other specimen ID values and Processing Instructions (R-28927)
- Removed Harvest Date labels from Web LDMS reports (R-28947)
- Fixed error when clearing additive or sub add/der on reagent logging page (R-29275)
- Fixed date pickers to work in Chrome, IE, and Firefox (R-29278)
- Fixed barcode scanning flagging all specimens as invalid on the QA/QC screen (R-29294)
- Fixed ability to save a patient that has a primary sample co-enrolled on two or more of its enrollments (R-29328)
- PDB files to be included in the deployment package (R-29329)
- Fixed some shipment destination fields not being visible on validation error (R-29335)
- Fixed QuickAdd Template filtering erroneously including disabled templates (R-29350)
