Frontier Science is pleased to announce that many new enhancements have been made to LDMS for the web (version 7.0).
Here are a few highlights from these changes, as well as the full release notes.
Highlights
Acknowledgement of Training
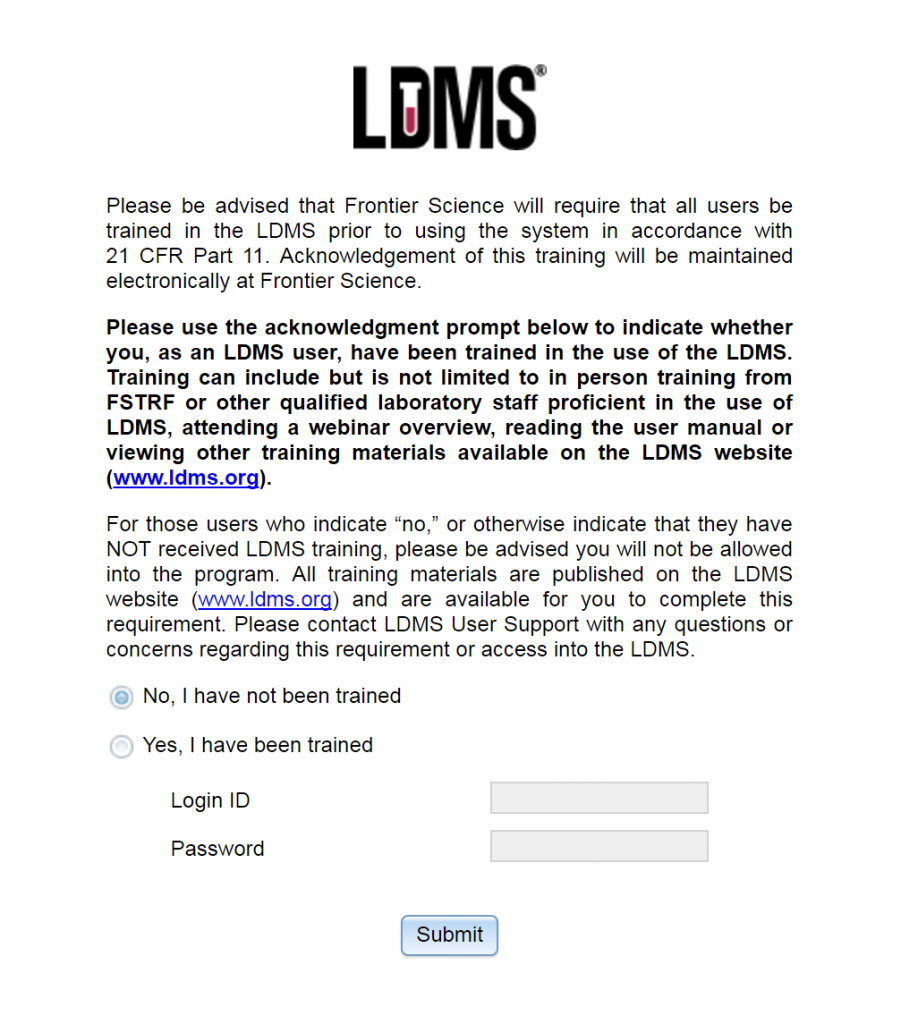
A new feature of LDMS for the Web is that when a new user logs into LDMS for the first time, they will be shown the following screen:
In our continuing effort to ensure our systems are in accordance with the requirements of 21 CFR Part 11, we have introduced the above acknowledgement agreement for LDMS users logging in for the first time. This agreement simply states that, by agreeing, you as a user are agreeing that you have been trained for use of LDMS. If a user has not been trained in the use of LDMS, they will not be permitted into the system until they have.
What constitutes training?
Listed in the acknowledgement screen above, “training” includes but is not limited to in-person training from Frontier Science Foundation or other qualified laboratory staff proficient in the use of LDMS, attending a webinar overview provided by a Frontier Science Foundation trainer, reading the LDMS user manual or viewing other training materials available on the LDMS website.
Settings Updates
A new Administration Setting for “Auto set frozen date and time when adding to storage” has been added. This setting allows the ability to set a frozen date and time when storing samples. If the user has enabled both this new feature and the auto-fill admin setting and is not shown the sample position picker, the frozen date and time will still be set to the current date and time at the lab when the new sample is stored. The processing tech initials will remain as the current value, or blank if none was entered.
Test Results Module Updates
Support for the Abbott RealTime HIV-1 RNA assay was added to Web LDMS Test Results Module. This includes Completed Test Run Report as well as a participant level report for Abbott RealTime HIV-1 RNA runs.
Workflow Improvements
There were many updates intended to improve the usability and workflow of Web LDMS. This section highlights only a select few.
Web LDMS will now generate and accept for upload a Cross LIMS manifest that includes 3 new fields: OtherSpecID, Time, and TimeUnit. The old Cross LIMS format is still supported, but this new format increases cross-compatibility greatly.
We have also added the ability to print labels from Quick Add directly, reducing the number of steps to do so and making the printing of labels even easier.
Report Updates
Further improvement have been made to correctly direct and help the user. We have added a report that displays data in the transaction log in a human-readable format.
Full Release Notes
There have been numerous improvements and fixes to this version of LDMS. The full list of changes in this release is below:
- Improved adherence to Web Standards by marking conditionally required fields with asterisks in addition to unconditionally required fields (R-20314)
- Added tooltips to the Sample Detail page for historical shipment storage samples (R-21384)
- Improved performance for un-import feature (R-25269)
- Reduced height of Web LDMS page header (R-27945)
- Fields from Cryopreservation form added to Customer Report Builder under Specimen Category (R-28169)
- Fixed issue with certain barcode labels not being able to be scanned (R-28287)
- Added new Administration Setting for “Auto set frozen date and time when adding to storage”. (R-28684)
- Added reagent logging fields to Custom Report Builder (R-28725)
- Web LDMS now takes user to existing record if attempting to add new Enrollment record (R-28757)
- Web LDMS now takes user to existing record if attempting to add new Visit record (R-28758)
- Project-specific labels are now shown in the UI in addition to standard ID1/ID2/ID3 nomenclature (R-28870)
- When printing labels for individual sample while viewing shipping container or storage tree on historical record, Web LDMS attempts to find appropriate live sample for the historical sample instead of using historical sample ID as the live sample ID (R-29002)
- Added report that displays the data in transaction log in a human-readable format (R-29127)
- Moving items in storage that would overflow destination now allows option for items to either be split with items able to fit being successfully moved, OR change the page order so the user may select a number of items that would fit in destination (R-29258)
- Error message when importing CSV files with invalid setup date reformatted to be clearer and easier to read (R-29310)
- Added ability to navigate specimen management grids with keyboard (R-29338)
- Quick Add Template List report shows whether or not a template is disabled (R-29356)
- Added filter criteria for Quick Add Template List report to choose to display only enabled or disabled templates (R-29357)
- Quick Add Templates on restricted groups have “Disabled” checkbox disabled (R-29375)
- Cryopreservation fields are correctly disabled when “Results Obtained” checkbox is not checked (R-29383)
- Reformatted tooltip text for “Unknown Condition” on invalid condition codes (R-29431)
- Quick Add Template with invalid subsamples can no longer be saved (R-29436)
- Fixed issue where some filters were not responding correctly to input (R-29530)
- Fixed “Mark To Ship” intended lab filter to accept enough characters for full name when typed for search by user (R-29533)
- Fixed security issue where password values were not masked in form data of logged errors. Retroactively masked password values in existing error log files (R-29659)
- Added Abbott RealTime HIV-1 RNA support to Web LDMS (R-29688)
- Fixed QA/QC status to correctly update on pending shipment screen (R-29689)
- Fixed overlapping UI issue where the navigation menu would shorten to two rows and overlap the bottom row when the browser window was too small to fit the whole menu on one screen (R-29716)
- Activated Clinic Field for MTN in Web LDMS (done retroactively as well) (R-29726)
- Filtering sample chooser converted to a paged grid to improve performance (R-29754)
- Security improvement made that now locks the inactive web page if two tabs in the same browser are opened to Web LDMS with different users logged in at the same time (R-29778)
- Updated range check on “Cryopreservation – Number of Vials Frozen” field to allow for numbers 0-X where X is the largest value allowed in the corresponding CDB field (R-29782)
- Added link for Reagent Logging page to the Welcome Page (R-29783)
- Inactive projects in LDMS set to non-exportable (ATN, IPREX, IRC, SHIMS, FACTS) (R-29807)
- Added Abbott Realtime HIV-1 Assay Completed Test Run Report to Web LDMS (R-29865)
- Added participant level report for Abbott RealTime HIV-1 RNA runs (R-29866)
- Disabled confirm position button after selecting last position to prevent duplicate form submissions (R-29880)
- Fixed issue where Clinic filter for
spec5.rptand others were not implemented (R-29893) - Web LDMS will now generate and accept for upload a Cross LIMS manifest that includes 3 new fields: OtherSpecID, Time, and TimeUnit (old Cross LIMS format is still supported) (R-29907)
- Improved database/network efficiency of filtering sample chooser (R-29968)
- Added ability to print labels from Quick Add (R-29988)
- Added new exportable project “mSTUDY” (R-29992)
- Fixed issue where some Global Specimen IDs were getting cut off when generating storage detail report (R-30048)
- Deactivated ID3 for AREAS, CHAVI, MTN, PHACS, and VTN in Web LDMS (already disabled in Windows LDMS) (R-30196)
- TaqMan Participant report now displays import date for received date field if available (R-30201)
- Custom reports date criteria now requires a date be entered or can be left as blank (R-30202)
- Updated error text when generating custom report with fields in “Sort By” grid, other (different) fields to the select list, and the “Distinct” checkbox is checked. Text is now clearer/more helpful (R-30204)
- Fixed potential security vulnerability to malicious links for opening links not maintained by Frontier Science Foundation (R-30215)
- Added new acknowledgement prompt/window to Web LDMS for new web users (R-30218)
- Fixed Web LDMS error that sometimes occurs when searching for a sample to add to a batch (R-30256)
- Fixed Clear Received Shipment Original Visit patch not completing successfully for one lab (R-30329)
- Improved clickable area for Specimen Management edit button to be easier to click (only an issue for certain browsers) (R-30363)
- Moving existing stored specimens into a storage contained now correctly invalidates the container (R-30366)
- Fixed “Delete” and “Cancel” buttons not displaying properly in Specimen Management when attempting to delete an aliquot that has been resulted in a run (R-30367)
- Fixed validation error message not displaying when saving a quick add template for a restricted project (R-30373)
- Fixed issue where Specimen Management barcode scan couldn’t select primary/aliquot (R-30379)
