Frontier Science Foundation is proud to announce the release of LDMS for the Web version 8.0.
A few highlights from the release are below, or you can jump to the full release notes.
Custom Report Builder Improvements
We’ve made several improvements to the Custom Report Builder feature:
- We’ve improved the error message when creating search criteria, providing more information and examples when an error occurs.
- Carrier and tracking number have been added as fields.
- Sample database ID has been added as a field (this is useful for older specimens that may be missing a specimen ID or global specimen ID.
- The speed of the Custom Report Build has been improved so reports can generate faster.
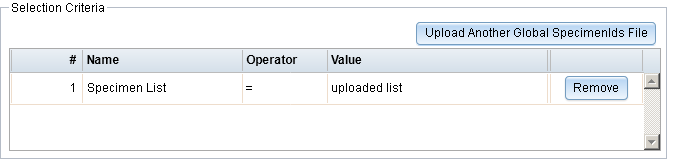
- You can now import a list of global specimen IDs to be used to generate a report.
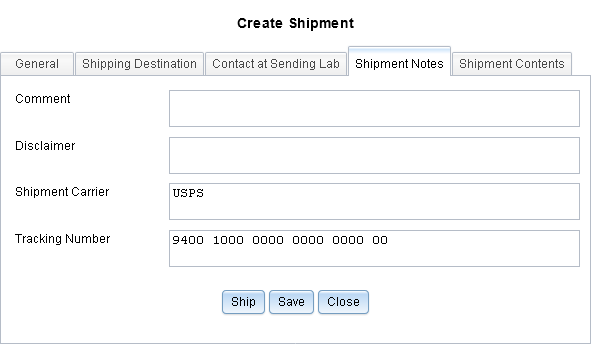
Added Support for Shipping Tracking Information
When creating shipments in LDMS, you can now add information about the shipment carrier and the shipment tracking number. This information is not included when the shipment when it is sent, so it is not received by the receiving laboratory.
Accessibility Improvements
Closely following accessibility standards such as WCAG and Section 508 is a key goal of LDMS development. We’ve made a number of improvements to LDMS 8.0 to make it easier for everyone to use. These changes include:
- Improved keyboard support for filters
- More use of ARIA attributes throughout the application
- Improvements to error message to make the application easier to understand
- Improvements to forms within the application to make them easier to use
Inactive Users Automatically Disabled
LDMS currently expires passwords after a certain number of days. To improve the security of LDMS, if users do not sign in for a certain amount of time, their accounts will now automatically become inactive as well.
If users with an inactive accounts attempts to sign in, they will be directed on the sign in page to contact User Support for assistance.
Full Release Notes
LDMS for the Web
- Added support for the ZIP 2.0 protocol R-32188
- Fixed issue where filters could not be selected by keyboard R-31956
- Fixed issue where Received Time was required for HPTN (this field is not required for HPTN in LDMS for Windows) R-31847
- Fixed issue where a Quick Add Template could not be modified if a primary specimen was removed from it R-31797
- Fixed issue that prevent the Specimen Log Report from generating under certain conditions R-31784
- Fixed issue where date and time were incorrectly converted between time zones when generating reports R-31780
- Improved error messages that can appear when using the Custom Report Builder R-31770
- Improved error message that can appear when the same user attempts to generate multiple reports R-31741
- Fixed issue where global specimen ID was cut off barcode label 20 R-31719
- Clinic field can now be set for HPTN specimens R-31669
- Fixed issue where specimens with a Harvest Date could not be deleted R-31621
- Added support for the BHP project R-31614
- Fixed issue where certain PHIA-specific validation rules were not applied when modifying a participant or enrollment R-31598
- Fixed issue where aliquot specimens could not be removed from Quick Add templates R-31596
- Fixed issue where ACTG/IMPAACT ID3 (SID) was not validated correctly when editing an enrollment R-31592
- The Transaction Log Report now shows seconds in addition to hours and minutes R-31577
- If users do not sign in for a set number of days, their account will not be disabled automatically R-31574
- Added Carrier and Tracking Number shipping fields to the Custom Report Builder R-31547
- Added support for new COBAS TaqMan and Abbott file formats R-31476
- Fixed issue where content was cut off from barcode label 9 R-31470
- In Storage Action Report, change New Location column to Current Location R-31439
- Fixed issue where the visit selection did not load when modifying an enrollment R-31429
- Fixed issue where when receiving a shipment in CSV format the shipping contain preview did not show specimens in the correct location R-31357
- Fixed issue where modifying the condition code of multiple specimens at the same caused all specimens to be marked as unavailable R-31328
- Corrected wording on Pending Shipments page to use Shipment Number instead of Batch Number R-31317
- Fixed issue where duplicate Target Not Detected results were found erroneously found in Abbott result files R-31279
- Fixed issue were specimens that were on a deleted Abbott test run could not be deleted R-31262
- Improved error message for CSV, SeraCare, and cross-LIMS shipments that do not contain any specimens R-31191
- Added support for non-logged specimens and controls in test results R-31130
- When shipping specimens for government projects, co-enrollments with local projects will no longer be included with the shipment file R-31108
- Improved wording of message that appears when loading a Quick Add template R-31010
- Fixed issue that allow Tech initials longer than 3 characters to be entered on some pages R-30998
- Added Sample Database ID as a field in the Custom Report Builder (this field for older specimens that may not have a global specimen ID or specimen ID R-30595
- Specimen ID is now displayed when selecting specimens to add to storage R-30524
- Fixed reports that did not have adequate space to display the current format global specimen ID R-30236
- Improved use of ARIA accessibility features R-29947
- Fixed issue where the title of some pages in the browser window did not match the title shown on the page R-29946
- End date is no longer a required field when using the Reagent Logging feature R-29823
- Improved usability of pages where the user can update data by allow the user to change the data directly instead of updating a separate form and clicking an update button R-29821
- Fixed issue where a page would no longer respond when invalid data was entered R-28842
- Added new Reagent Logging Report R-28724
- Added support for entered shipment tracking information to specimen shipments R-27873
- Fixed issue where a shipment with specimens that were added to a test result run could be un-imported R-27489
- Improved performance of Custom Report Builder R-25637
- Improved performance of Edit Enrollment page R-25626
- Improved performance of Edit Participant page – trim enrollment view model R-25625
- When adding or modifying a visit, ID3 values are now limited to those for the current participant R-21019
- Improved error message that appears when attempting to delete a storage item template that is currently in use. R-20326
- Add ability to select all specimens found in a search when storing specimens R-19534
- Add ability to import a list of global specimen IDs in Custom Report Builder R-16202
Account Manager
- Added ability to export event list as a CSV file R-31838
- Added filters to Events page R-31837
- Added laboratory ID associations to Accounts page R-31300
- Added account creation and modification dates to Accounts page R-31298
- Improved navigation menu by using drop-down menus R-30956
- When applying patches to a laboratory’s database, patches that are specific to other laboratory will no longer be displayed R-27453
